Evolution of subfunctionalization in metabolic genes Comparative physiology is a field that celebrates novelty, but intermediary metabolism is notoriously conserved, particularly amongst animals. Short-term regulation of existing enzymes can deal with changes in metabolic flux, and cells can produce more or less of the enzymes to deal with longer term changes. Over evolutionary time there is potential for the proteins themselves to change, yet many differences in primary structure are inconsequential. Against this background of conservation, there are examples where whole genome duplications enabled the evolution of functional divergence in paralogous genes/proteins of animals. In this seminar I will discuss examples where paralogous genes/proteins show lineage-specific patterns of subfunctionalization. That is, genes/proteins that may be orthologous in origin have evolved differences in function, with important consequences for the evolution of energy metabolism.
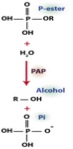
Feeding hungry plants: exploring the role of cell wall-targeted purple acid phosphatases in Arabidopsis phosphate acquisition Phosphorus (P) is a limiting macronutrient that roots must assimilate from the soil as soluble inorganic phosphate (Pi; H2PO4-). The most abundant P fraction of many soils exists as organic P-monoesters unavailable for root uptake until hydrolyzed by secretory purple acid phosphatases (PAPs). Plant PAPs belong to a relatively large multigene family whose specific functions in P-metabolism are poorly understood. Purification, characterization, and identification (via peptide sequencing) of native PAP isozymes upregulated by Pi-starved suspension cell cultures of Arabidopsis thaliana has been complemented by studies of the corresponding loss-of-function pap mutant seedlings. This approach has pinpointed the predominant Pi-starvation inducible (PSI), cell wall (CW) targeted PAP isozymes (AtPAP12, AtPAP17, AtPAP25, and especially AtPAP26) that appear to facilitate Arabidopsis Pi-acquisition from extracellular P-esters. AtPAP26 is secreted into the CW as a pair of differentially glycosylated ‘glycoforms’ (AtPAP26-CW1 and -CW2). AtPAP26-CW2 is a high mannose glycoform that interacts with a PSI, CW lectin (curculin). This is the first time that glycoforms or a lectin have been implicated in the plant Pi starvation response. The objective of my PhD thesis is to further our understanding of biochemical and functional properties of CW-targeted PAPs of Pi-deprived Arabidopsis, particularly AtPAP26-CW1 and –CW2, as well as AtPAP17, and AtPAP25. Overall, these studies are relevant to applied efforts to bioengineer crops that are more efficient at acquiring and using Pi, urgently needed to reduce the massive overuse of non-renewable and polluting Pi-containing fertilizers in agriculture. 11:30-12:30 BioSci Rm. 3110
Characterizing the calcium dependent regulation of plant myosins Our lab is primarily interested in understanding how plants use calcium signal transduction to regulate various cellular events. In animal cells, links between calcium signalling and cytoskeletal activity have been well documented. For example, a major component of the animal cytoskeleton is the acto-myosin complex, where myosin motor-domain proteins “walk” processively along actin filaments and function in a range of important events including muscle contraction, chromosomal rearrangements, and many other processes. Calcium signalling is linked to animal myosin function through the binding of the canonical calcium sensor, calmodulin, to myosin neck regions where it functions as part of the ‘lever’ mechanism in myosin walking. In plants, much less in known about the biochemical properties and physiological roles of myosins. Arabidopsis possesses 17 myosin isoforms divisible into two classes based upon predicted structural differences; class VIII and XI. Although these plant myosins have not been well studied, emerging evidence suggests roles in events such as organelle remodeling and movement, gravotropic response, immune response, and cell expansion. Thus, myosins represent an interesting link between cytoskeletal activity and calcium signal transduction in plants. Surprisingly, evidence for an interaction between calmodulin and myosins in plants is not well established. My project aims to explore questions addressing how plant myosins use calcium sensors to regulate their activity. I will outline my research plan and progress to date drawn from a variety of biochemical, genetic, and physiological approaches. 11:30-12:30 BioSci Rm. 3110
|
Archives
February 2021
|